Project Report For Calcium Carbide Manufacturing
Introduction
Project report for Calcium Carbide Manufacturing is as follows.
Calcium carbide is used in a variety of sectors, including calcium cyanamide, oxyacetylene welding, medicines, sodium cyanide for gold ore treatment, food, synthetic rubber, plastic, acetic acid, and PVC.
Fertilizer businesses utilise it as a fundamental raw ingredient. It’s also employed as a desulfurizing agent, a dehydrating agent, and a reducing agent. It is one of the most often demanded and manufactured chemicals due to its extensive chain of relevance. The trend rate of growth is discovered to be 3.53 percent each year, as it is necessary in large quality.
Calcium carbide has the molecular weight of 64.0992 g/mol and the nominal formula of CaC2. The pure substance is colourless, however, depending on the quality, most samples range in colour from black to greyish-white.
Project Report Sample On Calcium
Carbide Manufacturing
Get Completely Custom Bankable Project Report
It has a density of 2.22 g/cc, melts at 2160 °C, and boils at 2300 °C (in an inert environment), when it decomposes. It is primarily used in the manufacturing of acetylene and calcium cyanamide, CaCN2. Calcium carbide is made in an electric-arc furnace at a temperature of around 2000°C from a combination of CaCO3 and coke (carbon).
Because classical combustion cannot achieve the high temperature necessary for this reaction, the process is carried out in an electric-arc furnace using graphite electrodes. The calcium carbide product generated is typically 80–85 percent calcium carbide by weight.

The carbide is crushed into little lumps ranging in size from a few millimetres to 50 millimetres. In the finer fractions, the contaminants are concentrated. The amount of acetylene formed during hydrolysis is used to determine the product’s CaC2 concentration.
The coarser fractions content requirement in the United States, for example, is 295–300 l/kg. Impurities in the carbide include phosphide, which when hydrolyzed to generate HCtriple bondCH, i.e. acetylene, releases PH3 (a toxic gas).
This reaction was a key aspect of the chemical industrial revolution, and it was made possible in the United States by enormous amounts of inexpensive hydroelectric power created at Niagara Falls around the turn of the century.
Market Potential Of Calcium Carbide Manufacturing
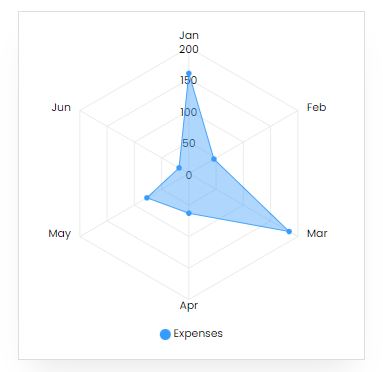
Expenses
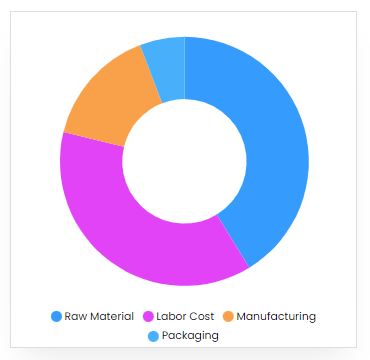
Product Cost Breakup
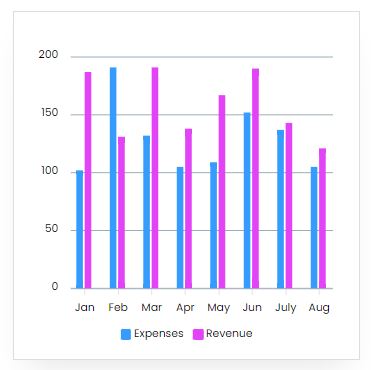
Reveneue Vs Expenses
Market Trend
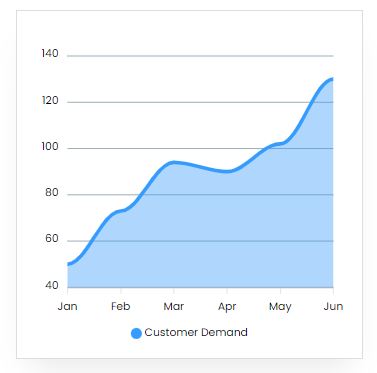
From 2021 to 2030, the worldwide calcium carbide unsaturated polyester resins market is expected to increase at a CAGR of 5.0 percent, from $11.3 billion in 2020 to $18.2 billion by 2030.
When pure, calcium carbide (CaC2) is a colourless, transparent substance with a molecular weight of 64.0992 g/mol. White calcium carbide may be made in a laboratory by thermal breakdown of pure calcium cyanimide in the presence of carbon under a vacuum.
The characteristics of pure calcium carbide vary, and they have been found by extrapolating from grades achieved on high-purity commercial carbide. Calcium cyanamide is made by passing nitrogen through powdered calcium carbide in an electric arc furnace, which is one of CaC2’s uses. During the projected period, the calcium carbide market is likely to be driven by a rise in demand for calcium cyanamide.
Calcium carbide is made at a factory using calcium oxide and coke. It is used in the chemical industry for a number of purposes, including the production of acetylene gas and the manufacture of acetylene in carbide lamps, fertiliser manufacturing, and steelmaking. Steel is utilised in construction because of its outstanding features of ductility and durability. It assists in the development of structures that are earthquake-resistant.
The rising use of high-speed trains in countries like China, Japan, and India has led in the creation of special rails, which has resulted in a rise in the use of carbon steel. As a consequence, the calcium carbide market is expected to increase faster than the overall market over the projected period.
