Project Report For Lead Acid Battery Manufacturing
Introduction
Project report for Lead Acid Battery Manufacturing is as follows.
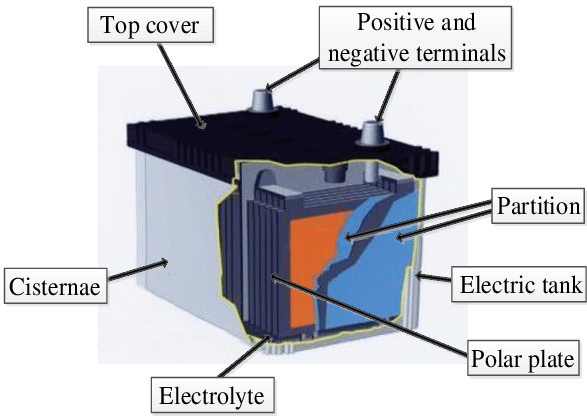
Lead alloy ingots and lead oxide are used to make the lead battery. It consists of two sulphuric acid-immersed plates with chemically different leads. The positive plate is composed of lead dioxide (PbO2), whereas the negative plate is composed entirely of pure lead. When these two plates are submerged in dilute sulfuric acid, the nominal electric potential between them is 2 volts.
All lead-acid batteries have the same potential. As illustrated in the illustration, a 12-volt lead-acid battery is made up of six cells linked in series and housed in a sturdy plastic housing. The quantity of lead dioxide on the positive plate, the amount of sulfuric acid in the battery, and the amount of spongy lead on the negative plate all affect the battery’s capacity.
Project Report Sample Of Lead
Acid Battery Manufacturing
Get Completely Custom Bankable Project Report
The sulphate ions in the electrolyte interact with the positive and negative plates during the discharging process, forming lead sulphate on them. As a consequence, the specific gravity of the electrolyte decreases in proportion to the charge applied to the load.
The cycle is reversed during the charging process, with lead sulphate and water being transformed to lead, lead oxide, and sulphuric acid electrolyte by an external charging source. Because this process is reversible, lead acid batteries may be drained and recharged several times.
The sulphate ions in the electrolyte interact with the positive and negative plates during the discharging process, forming lead sulphate on them. As a consequence, the specific gravity of the electrolyte decreases in proportion to the charge applied to the load.
The cycle is reversed during the charging process, with lead sulphate and water being transformed to lead, lead oxide, and sulphuric acid electrolyte by an external charging source. Because this process is reversible, lead acid batteries may be drained and recharged several times.
Market Potenial Of Acid Battery Manufacturing
Expenses
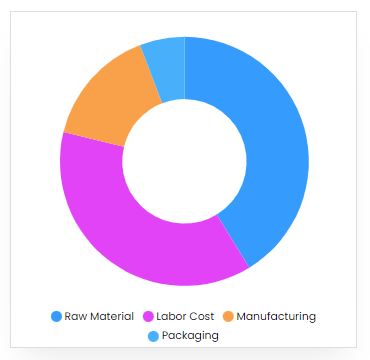
Product Cost Breakup
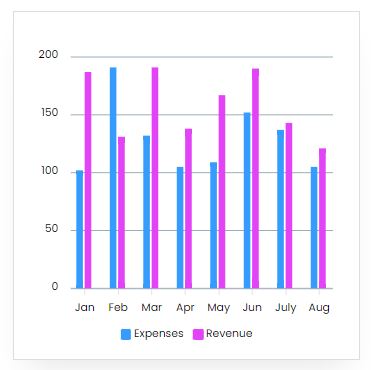
Reveneue Vs Expenses
Market Trend
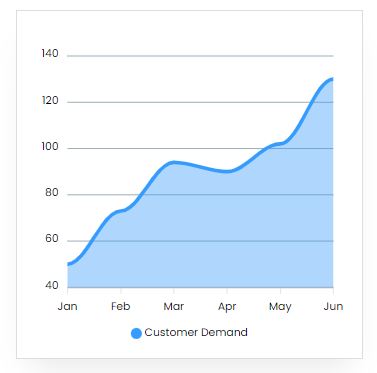
The worldwide lead–acid battery market was valued at $39.7 billion in 2018, and is expected to increase at a CAGR of 5.24 percent from 2019 to 2026, to reach $59.7 billion.
A lead–acid battery is made up of a sponge metallic lead anode, a lead-dioxide cathode, and a sulphuric acid solution electrolyte. It is the world’s first commercially available battery. It contains hazardous lead, although it may be recycled.
The worldwide lead–acid battery market is rapidly expanding throughout the world, with a CAGR of 5.2 percent expected during the projected period. Growing SLI applications in the automobile sector, increase in renewable energy output, and rising demand for energy storage devices are some of the causes driving up demand for lead–acid batteries.
As the telecom sector expands in nations like the United States, Brazil, India, and the United Kingdom, there is a growing need for UPS systems as a backup power source, resulting in a higher usage of lead–acid batteries as a cost-effective energy source. However, the rising use and popularity of lithium-ion batteries stifles market growth to a significant amount.
Lithium-ion batteries are the most common replacements for lead–acid batteries because they provide more advantages. As a result, over the forecast period, these factors are expected to provide a challenge to the worldwide lead–acid battery market’s growth. Major technological companies such as Google and Samsung are planning to expand their data centres in order to reduce data complexity.
As a result, the demand for UPS systems in data centres throughout the world is increasing, which might contribute to significant development possibilities for the worldwide lead–acid market during the projected period. The primary worldwide lead–acid battery market trend noted in recent years is the expansion of data centres and the associated need for large weight lead-acid batteries.

