Project Report For Oxalic Acid
Introduction
Project report for Oxalic Acid is as follows.
The organic compound oxalic acid has the chemical formula C2H2O4. It is a clear, crystalline material that dissolves in water to produce a clear solution. The acronym HOOCCOOH, which reflects the fact that it is the simplest dicarboxylic acid, is used to represent it.
It has a much stronger acid than acetic acid. The conjugate basic of oxalic acid, oxalate (C2O2-4), is a chelating agent for metal cations and a reducing agent[6]. Oxalic acid often occurs as a dehydrate with the chemical formula C2H2O4•2H2O. Excessive oxalic acid consumption or protracted skin contact may be harmful.
Oxalic acid is created by oxidizing sugar with nitric acid, sulfuric acid, and vanadium pentoxide while also acting as a catalyst. Nitric acid is well known for being able to oxidize a variety of carbohydrates, including glucose, sucrose, starch, dextrin, cellulose, and others, to form oxalic acid. oxalate-containing barks that were bought from the forest service.

Molasses was initially preheated to a temperature of 65.5 degrees Celsius from 37 degrees. The material was subsequently injected into the reactor. At the same time, nitric acid and a catalyst called vanadium pentaoxide were fed into the reactor.
This well-blended concoction was given two to three hours to react. After two to three hours, oxalic acid, unreacted molasses, unreacted nitric acid, and nitrogen oxide were produced. Oxalic acid, unreacted molasses, unreacted nitric acid, and vanadium pentoxide depart the CSTR’s bottom section and are further separated.
During this process, the vanadium pentoxide is initially separated using a filter. Oxalic acid and mother liquor (unreacted nitric acid and molasses) are separated using a two-stage process. The first stage involves adding filtered solution into a crystallizer to create mother liquor and oxalic acid crystals, which are then separated using a centrifuge.
Oxalic acid is re-crystallized after being separated in order to eliminate inclusions by adding hot water inside a crystallizer containing these oxalic acid crystals (the process by which a solvent particle becomes caught inside a crystal). After being once more separated from the mother liquor, oxalic acid crystals are put in a dryer to remove any moisture from their surface.
Get Completely Custom Bankable Project Report
The nitrogen oxide gas that escapes from the top surface of the CSTR was directed into a compressor to boost pressure and to a steam, heater to increase temperature because it cannot be released directly into the atmosphere due to its potential to create air pollution.
Then, this gas was transferred to a fluidized-bed reactor, where it was converted to nitrogen dioxide gas in the presence of an Al203 catalyst. After that, a cyclone separator separates Al203. Following this procedure, nitric acid and nitrogen oxide gas was produced in an absorber by allowing nitrogen dioxide gas to react with water sprayed inside the absorber.
Market Potential Of Oxalic Acid
The oxalic acid market is anticipated to reach a value of approximately US$ 945.3 Million in 2022. Oxalic acid is being used more often for a wide range of applications, and the market will grow at a moderate CAGR of 3.9% and surpass US$ 1.19 Billion by 2028.
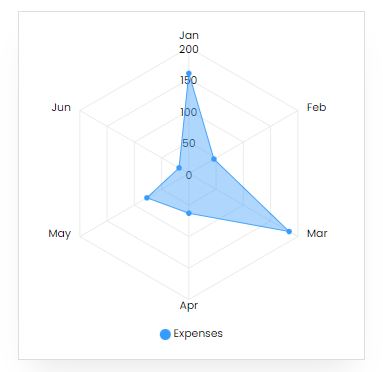
Expenses
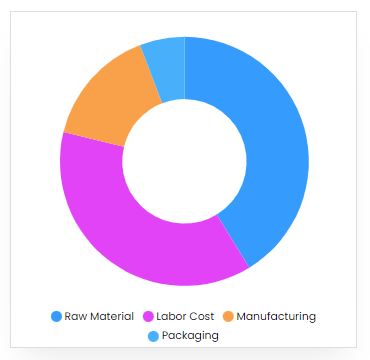
Product Cost Breakup
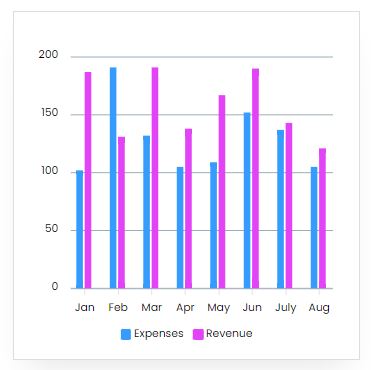
Reveneue Vs Expenses
Market Trend
Oxalic acid is a colorless, crystalline substance with a sharp, sour flavor. Oxalic acid can be hazardous in large doses. It is a member of the carboxylic acid family. Oxalic acid is soluble in water, alcohol, and ether. Oxalic acid is a typical cleaning or bleaching agent.
The mordant oxalic acid is used in the dying process. Oxalic acid is used by pharmaceutical businesses to dilute or purify products. The smelting of rare earth makes considerable use of oxalic acid.
Textile and wood industries both utilize oxalic acid as a bleaching agent. Both the water treatment industry and the metal treatment industry depend on oxalic acid to eliminate rust from metals. It was anticipated that 220 million metric tonnes of oxalic acid would be produced by the end of 2019.
Oxalic acid is used for bleaching and cleaning in many commercial and domestic applications, which is encouraging for the market’s predicted growth.
Oxalic acid is required due to the industry’s growing concern regarding sterilizing. However, its use in the home and commercial settings may lessen the demand for oxalic acid because it is dangerous and corrosive.
Based on application, the global oxalic acid market is segmented into the following groups: Pharmaceuticals, the rare earth industry, textiles, and others (Cleaning, and Surface Dust Removal.)
The three manufacturing procedures utilized to make oxalic acid are the nitric acid oxidation process, the sodium formate dehydrogenation method, and the dialkyl oxalate hydrolysis process.
