Feasibility Report On Sodium Silicate
Sodium silicate is a versatile chemical compound made from sodium, silicon, and oxygen. It’s commonly known as “liquid glass” due to its liquid form. Used in various industries, it acts as an adhesive, binder, and sealant. It finds applications in detergents, construction, automotive, and arts due to its adhesive and protective properties.
Introduction
Feasibility Report For Sodium Silicate.
Sodium Silicate is a colorless chemical composed of sodium and silica oxides. It has a variety of chemical formulas that differ in the amounts or ratios of sodium oxide (Na2O) and silicon dioxide or silica (SiO2). It is water soluble and is made by reacting silica sand and sodium carbonate at high temperatures ranging from 1200 to 1400 degrees Celsius. Water glass is an aqueous solution of sodium silicate.



Sodium Silicate is an inorganic compound with unique characteristics that are not found in other alkaline salts. Sodium silicates are also referred to as water glass or liquid glass. In both neutral and alkaline solutions, sodium silicate is stable. Silicate ions combine with hydrogen ions in acidic liquids to generate silica acid, which when heated and roasted makes silica gel, a hard, glassy solid. When melted, sodium carbonate and silicon dioxide react to generate sodium silicate and carbon dioxide. The soluble’s qualities and various functional characteristics might be employed to efficiently and economically handle the majority of the difficulties that arise in some chemical and industrial processes.
Sodium Silicate is used in the production of soaps, detergents, and silica gel. It’s a cement, binder, filler, and adhesive. Also used as a wall coating, in concrete, as a fire retardant, and as a sealer. It’s also used to keep eggs and wood fresh. Sodium Silicate is also used in the textile and pharmaceutical industries. In liquid form, neutral sodium silicate is acceptable for use in medicinal and toilet preparations. Silica sand and Sodium carbonate are the two principal raw materials used in the manufacture of sodium silicate. The proportions of these two raw materials will vary depending on the ratio of Na2O and SiO2 required in the final product.

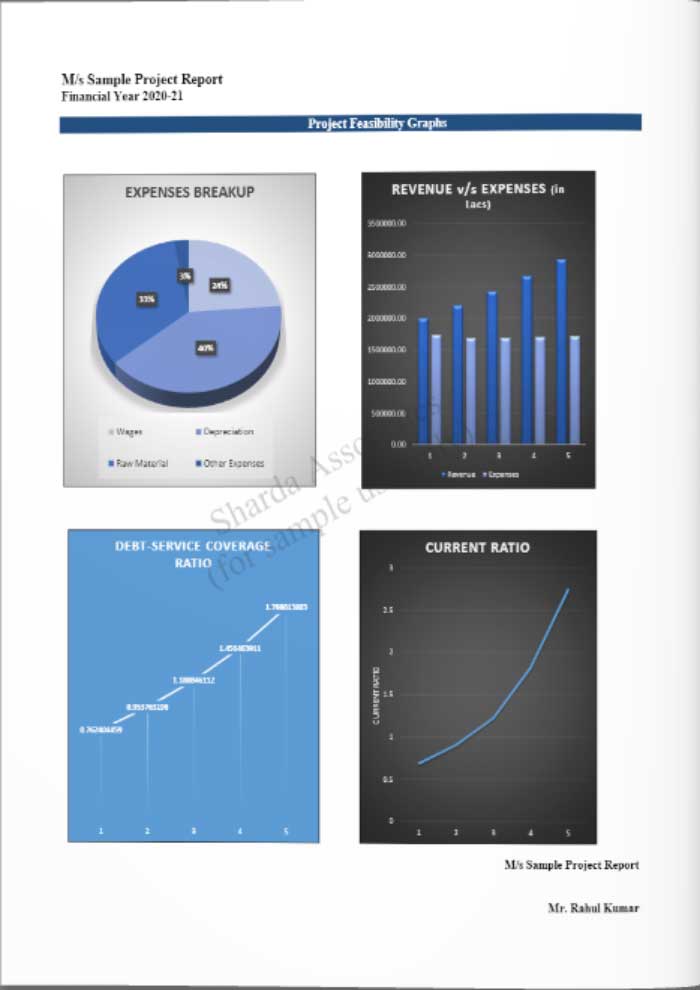
Feasibility Report Sample On Sodium Silicate


Market Strategy of Sodium Silicate
The global sodium silicate market size reached US$ 7.5 Billion in 2022. Looking forward, IMARC Group expects the market to reach US$ 10.0 Billion by 2028, exhibiting a growth rate (CAGR) of 4.65% during 2023-2028. The rapid industrialization activities, implementation of stringent environmental regulations, increasing urbanization and infrastructural development, widespread product utilization in water treatment, and increasing product adoption as an adhesive in the automotive manufacturing are some of the major factors propelling the market.

Water glass, also known as sodium silicate, is an inorganic substance composed of silicon, sodium, oxygen, and water. Its synthesis requires the high-temperature melting of silica sand and sodium carbonate. Sodium silicate has high alkalinity, is water soluble, and is sticky. It’s commonly utilized in detergents, paper, textiles, construction, water treatment, soil stability, fire prevention, and adhesives. Sodium silicate is a low-cost, flexible, and environmentally harmless substance with string-bonding properties and acid and water resistance.



