Project Report For Medicine Factory
Introduction
Project report for Medicine Factory is as follows.
The pharmaceutical sector includes both public and commercial entities that discover, produce, and manufacture medicines and remedies (pharmaceuticals).
Thousands of years after intuition and trial and error led humans to believe that plants, animals, and minerals contained medicinal properties, the modern era of the pharmaceutical industry of compound isolation and purification, chemical synthesis, and computer-aided drug design is thought to have begun in the 19th century.
Project Report Sample On Medicine Factory
Get Completely Custom Bankable Project Report
The integration of study in domains like chemistry and physiology in the twentieth century improved our understanding of basic drug-discovery processes.
A clinician is a health practitioner who works directly with patients in a hospital or other healthcare setting. Nurses, doctors, psychotherapists, and other professionals are among the clinicians.

Identifying novel therapeutic targets, obtaining regulatory approval from government bodies, and improving drug research and development procedures are just a few of the issues that the pharmaceutical business is facing today. The pharmaceutical industry’s continuous growth and advancement is critical to the global control and elimination of illness.
Not all medical experts are clinicians. Researchers and laboratory workers are not considered clinicians since they do not work with patients.
The physician assesses the individual using knowledge gathered through training, research, and experiences, as well as clinical judgement, with the objective of diagnosing, treating, and preventing disease
Market Potential Of Medicine Factory
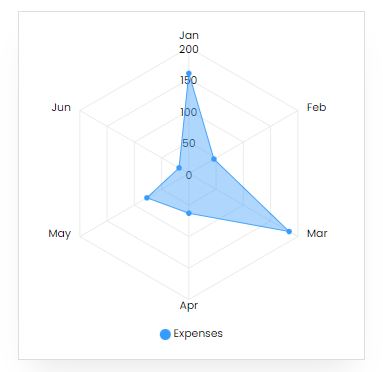
Expenses
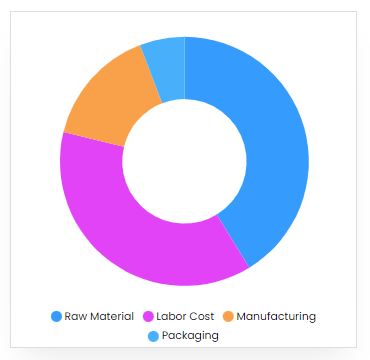
Product Cost Breakup
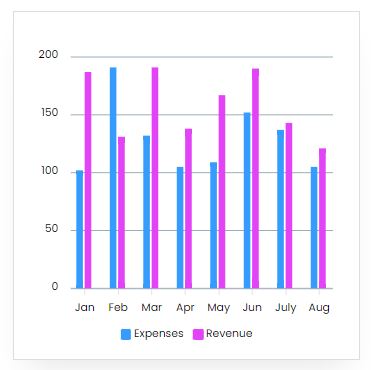
Reveneue Vs Expenses
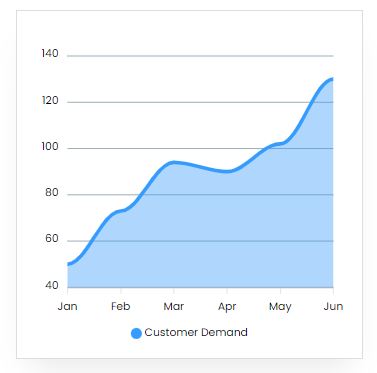
Market Trend
The worldwide pharmaceutical manufacturing market was worth USD 405.52 billion in 2020, and it is predicted to increase at an annual pace of 11.34 percent from 2021 to 2028.
With the introduction of new technology and more cost-effective and efficient manufacturing methods, the pharmaceutical landscape has experienced a significant upheaval. Furthermore, increased investment flow in this domain has had a beneficial influence on market growth.
Furthermore, in this market, single-use disposable solutions have gained traction and have supplanted open transfer production procedures. In addition, the paradigm change toward integrated, smart, data-rich paperless operations has resulted in error-free and exact output. Drug manufacture has accelerated as a result of these continuous improvements.
Consistent advancement in the field of customised medicine has opened up a slew of new options for treating a variety of ailments, as well as the creation of patient-centric models. As a result of this breakthrough, the development of complicated medications and autologous patient-centric therapies is shifting from big quantities to smaller batches. This has prompted manufacturers to rethink their supply chains in order to better match with the patient-centered health-care system.
The medication manufacturing techniques are projected to be fueled by an increase in drug approvals by regulatory agencies. The FDA, for example, authorised 59 medications in 2018, 49 pharmaceuticals in 2019, and 15 drugs until April 2020. In addition, a huge number of current clinical trials have offered several market development potential.
